Embark on a scientific odyssey with Balancing Chemical Equations Chapter 7 Worksheet 1, where the intricacies of chemical reactions unfold. This comprehensive guide unveils the fundamental principles of balancing equations, empowering students with the tools to decipher the language of chemistry.
Delving into the heart of the matter, this worksheet presents a meticulously crafted array of practice problems, ranging from unbalanced to partially balanced equations. Each problem is designed to challenge students’ understanding, fostering a deeper comprehension of the balancing process.
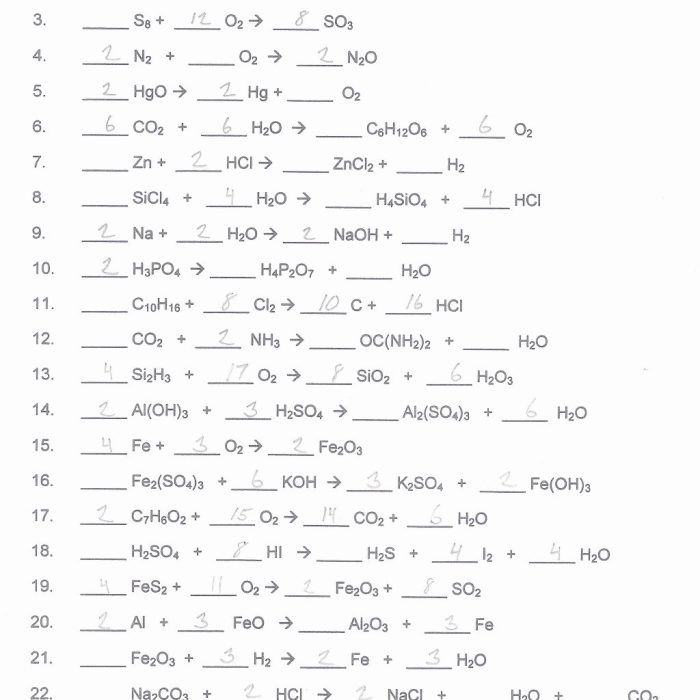
Balancing Chemical Equations: Balancing Chemical Equations Chapter 7 Worksheet 1

Balancing chemical equations is a crucial aspect of chemistry that involves adjusting the coefficients of reactants and products to ensure that the number of atoms of each element is equal on both sides of the equation. This process is essential for understanding the stoichiometry and predicting the products of chemical reactions.
Basic Rules and Steps
Balancing chemical equations follows specific rules and steps:
- Identify the unbalanced equation and count the number of atoms of each element on both sides.
- Adjust the coefficients of one reactant or product at a time, starting with the element that appears in the most compounds.
- Multiply the coefficients of all the atoms in the compound by the same factor.
- Continue adjusting coefficients until the number of atoms of each element is equal on both sides.
- Check the coefficients of all reactants and products to ensure that they are in the lowest whole-number ratio.
Methods for Balancing Chemical Equations
There are several methods for balancing chemical equations:
- Inspection method:Suitable for simple equations with a few reactants and products.
- Half-reaction method:Used for redox reactions, where the reaction is divided into two half-reactions.
- Oxidation-reduction method:Similar to the half-reaction method, but focuses on the transfer of electrons.
Troubleshooting Common Errors
Common errors in balancing chemical equations include:
- Changing the subscripts of atoms, which alters the chemical formula.
- Not adjusting the coefficients of all atoms in a compound.
- Using fractional coefficients, which are not allowed.
- Balancing the equation for one element but neglecting others.
Applications of Balancing Chemical Equations, Balancing chemical equations chapter 7 worksheet 1
Balancing chemical equations has practical applications in chemistry, such as:
- Predicting the products and stoichiometry of reactions.
- Designing chemical experiments and calculating the required amounts of reactants.
- Understanding the mechanisms and energetics of chemical reactions.
Quick FAQs
What is the significance of balancing chemical equations?
Balancing chemical equations is crucial for understanding the stoichiometry of reactions, ensuring that the number of atoms of each element on the reactants’ side matches the number on the products’ side. This allows for accurate predictions of reaction products and the calculation of quantitative relationships between reactants and products.
Can you explain the inspection method for balancing equations?
The inspection method involves systematically adjusting the stoichiometric coefficients of reactants and products until the number of atoms of each element is identical on both sides of the equation. This method is particularly useful for simple equations with few reactants and products.