In which of the following reactions will kc kp – In which of the following reactions will Kc = Kp? This question arises in the realm of chemical equilibrium, where understanding the relationship between equilibrium constants is crucial. This article explores the concept of equilibrium constants, Kc and Kp, their relationship, and the specific conditions under which they are equal.
Equilibrium constants are quantitative measures of the extent to which a chemical reaction proceeds towards completion. Kc, the equilibrium constant in terms of molar concentrations, is applicable to reactions in solution, while Kp, the equilibrium constant in terms of partial pressures, is suitable for reactions involving gases.
The relationship between Kc and Kp is established through the ideal gas law, and the equality of Kc and Kp holds true only under certain assumptions and limitations.
Equilibrium Constant (Kc) and Equilibrium Partial Pressure Constant (Kp)
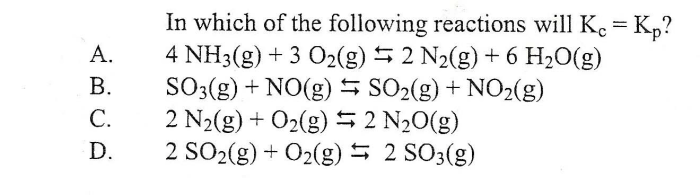
In a chemical reaction, the equilibrium constant is a value that describes the extent to which the reaction proceeds towards completion. There are two types of equilibrium constants: the equilibrium constant for reactions in solution (Kc) and the equilibrium partial pressure constant for reactions involving gases (Kp).
Kc is defined as the ratio of the concentrations of the products to the concentrations of the reactants, each raised to their stoichiometric coefficients. Kp, on the other hand, is defined as the ratio of the partial pressures of the products to the partial pressures of the reactants, each raised to their stoichiometric coefficients.
The relationship between Kc and Kp is given by the following equation:
Kp = Kc(RT)^Δn
where R is the ideal gas constant, T is the temperature in Kelvin, and Δn is the change in the number of moles of gas between the reactants and products.
Reactions Involving Gases
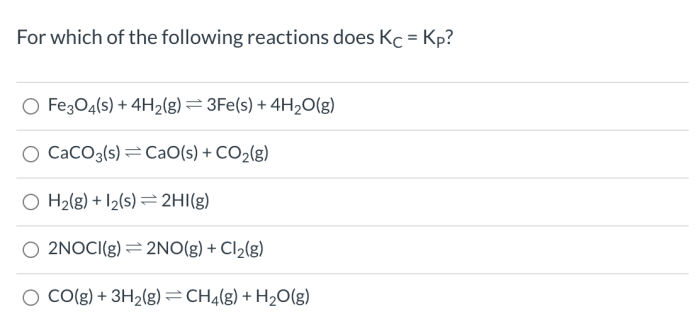
Kp is more appropriate to use for reactions involving gases because it takes into account the partial pressures of the gases, which are directly related to their concentrations.
The assumptions and limitations of using Kp for gas reactions are as follows:
- The gases behave ideally.
- The volume of the reaction vessel is constant.
- The temperature is constant.
To calculate Kp from Kc, the following equation can be used:
Kp = Kc(RT)^Δn
To calculate Kc from Kp, the following equation can be used:
Kc = Kp(RT)^-Δn
Reactions in Solution
Kc is more appropriate to use for reactions in solution because it takes into account the concentrations of the reactants and products in solution.
The assumptions and limitations of using Kc for reactions in solution are as follows:
- The solution is dilute.
- The temperature is constant.
- The ionic strength of the solution is constant.
To calculate Kc from Kp, the following equation can be used:
Kc = Kp(RT)^-Δn
To calculate Kp from Kc, the following equation can be used:
Kp = Kc(RT)^Δn
Temperature Dependence of Kc and Kp
The temperature dependence of Kc and Kp is given by the following equation:
ln(K) =
ΔH°/RT + C
where ΔH° is the enthalpy change of the reaction, R is the ideal gas constant, T is the temperature in Kelvin, and C is a constant.
The relationship between the enthalpy change of a reaction and the temperature dependence of Kc and Kp is as follows:
- If ΔH° is negative, then Kc and Kp will increase with increasing temperature.
- If ΔH° is positive, then Kc and Kp will decrease with increasing temperature.
Applications of Kc and Kp: In Which Of The Following Reactions Will Kc Kp

Kc and Kp have a wide range of applications in various fields such as chemistry, engineering, and environmental science.
Some specific examples of how Kc and Kp are used to solve real-world problems include:
- Predicting the extent to which a reaction will proceed towards completion.
- Determining the equilibrium concentrations of reactants and products.
- Calculating the solubility of a gas in a liquid.
- Designing chemical reactors.
- Predicting the environmental impact of chemical reactions.
Common Queries
What is the difference between Kc and Kp?
Kc is the equilibrium constant expressed in terms of molar concentrations, while Kp is the equilibrium constant expressed in terms of partial pressures.
Under what conditions are Kc and Kp equal?
Kc and Kp are equal when the reaction involves ideal gases and the volume of the system is constant.
How can I calculate Kp from Kc?
Kp can be calculated from Kc using the ideal gas law: Kp = Kc(RT)^(Δn), where R is the gas constant, T is the temperature, and Δn is the change in the number of moles of gas in the reaction.